New technology
SCALE UP PRODUCTION OF THE TWO LEADING PEPTIDES, PHC279 AND PHC949
PHC279 is a peptide derived from the Innatus 3G family within the PREtec platform. The Innatus 3G family has the potential to deliver multiple products that serve as plant vaccines to protect against crop diseases such as Asian soybean rust. Vaccinated plants also show better growth, strength, and yields. As a representative peptide, PHC279 has shown remarkable activity in multiple crops; it is but the first of many products from the Innatus 3G family.
Building on our pilot production experience with PHC279, the Seattle R&D team’s primary focus in 2022 was on supporting the scale-up manufacturing operations. This included fermentation, downstream processing and final formulation at a scale and consistency capable of supporting product launch and future growth, and at a cost to deliver attractive margins in market.
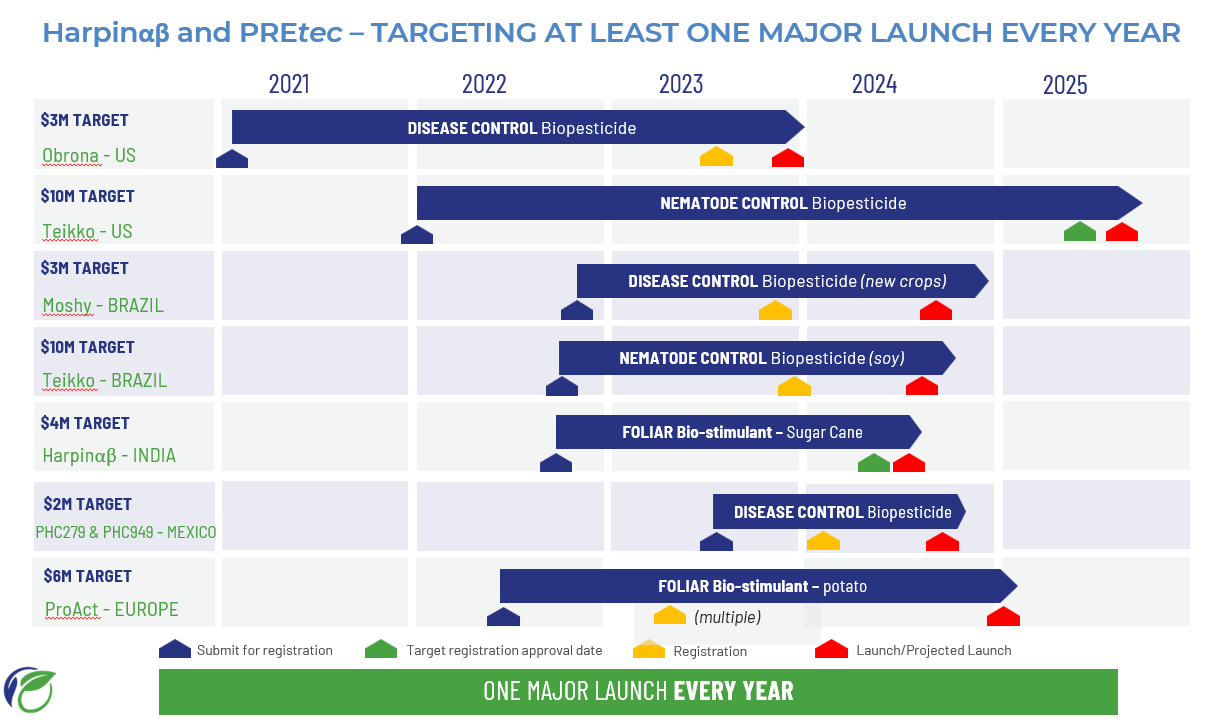
During the year, we achieved great success in improving yield and optimizing production methods, transferring a full production process for a third-party manufacturer. We sought and secured a reliable, cost-effective third-party manufacturer and successfully transferred the production methods for scale up production from 2-litre to 30-litre, and from there to commercial 5,000-litre fermentation tanks. The product produced from this large-scale production was supplied in 2022 to Brazil as Saori™, for the seed treatment of soybeans. In 2023, further refinements to the manufacturing conditions have resulted in further improvements in the cost of goods.


PHC949 is a peptide developed from the T-Rex 3G family of PREtec, which enables us to develop products specifically for the activation of plants’ natural defences against nematode infection. Nematodes are a major class of soil pests for both row crops and specialty crops. In addition to protection from nematodes, vaccinated plants also show great resistance to abiotic stress such as drought.
Remarkably for a plant response elicitor, PHC949 has consistently shown performance comparable to conventional chemicals in field trials. This is remarkable efficacy for a biological product, with its benign toxicological profile.
For PHC949, our R&D team focused on the development of production methods and continued work on scaling up manufacture. Great success was achieved in improving yield and scaling up from a 2-litre bench top fermenter to 30-litre tanks and transferring the method to our chosen contract manufacturer where the product is now being successfully produced at 5,000L, commercial-scale production to support the commercial launch in Brazil in 2024.